Introduction |
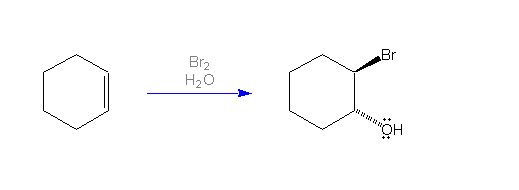 |
Alkenes react with halogens (Cl2,
Br2 and I2) in aqueous solution (or in the
presence of water) to form 2-haloalcohols or "halohydrins". The
reaction takes place with anti-addition. In the case of a cyclic alkene, the resulting halohydrin has the halogen and hydroxyl group
trans to one another. This mechanism is illustrated by the
addition of bromine and water across the carbon-carbon double bond of
cyclohexane to give trans-2-bromocyclohexanol. Consider the 3D
structure of the product on the right.
What positions do the bromine and hydroxyl
group occupy in this low enery conformation?
|
| |
|
|

