|
1. What is the ground-state electronic configuration of a fluoride anion (fluorine: atomic number 9)?
(A) 1s22>s22p2
(B) 1s22s22p5
(C) 1s2s22p6
(D) 1s2s22p7
2. Which of the following species possesses a formal charge?
(A) CCl4
(B) SiCl4
(C) AlCl4
(D) PCl3
3. Which of the following is trigonal planar?
(A) boron trifluoride, BF3
(B) methyl anion, CH3-
(C) methane, CH4
(D) ammonia, NH3
4. What is the approximate value of the H-C-H bond angles in methane, CH4?
(A) 90°
(B) 109.5°
(C) 120°
(D) 180°
5. Which of the following represents the shape of the 2s atomic orbital of carbon?
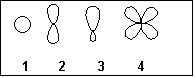
(A) 1
(B) 2
(C) 3
(D) 4
6. Which of the following represents an sp2 hybridized atomic orbital of carbon which overlaps with the 1s atomic orbital of hydrogen to form a C-H
s-bonding molecular orbital in ethene, H2C=CH2?
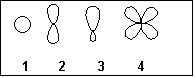
(A) 1
(B) 2
(C) 3
(D) 4
7. Which of the following statements is not true about the carbonate anion, CO32-?
(A) All of the oxygen atoms bear the same amount of charge
(B) All of the carbon-oxygen bonds are the same length
(C) The carbon atom bears the negative charge
(D) It is basic
8. Which atomic orbitals overlap to form the C-H
s-bonding molecular orbitals of ethene, H2C=CH2?
(A) Cp + Hs
(B) Csp + Hs
(C) Csp2 + Hs
(D) Csp3 + Hs
9. Which atomic orbitals overlap to form the C-H
s-bonding molecular orbitals of ethyne,
HCºCH?
(A) C2p + H1s
(B) Csp + H1s
(C) Csp2 + H1s
(D) Csp3 + H1s
10. What is the approximate Cl-C-Cl bond angle in dichloromethane, CH2Cl2?
(A)
90°
(B)
109.5°
(C)
120°
(D)
180°
|