|
1. What is the IUPAC name of the following compound?
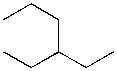
(A) 3-propylpentane
(B) 1,1-diethylpropane
(C) 3-ethylhexane
(D) isooctane
2. Which of the following Newman projections does not represent 2-methylhexane?

(A) 1
(B) 2
(C) 3
(D) 4
3. Which of the following cycloalkanes has the most ring strain?
(A) cyclopropane
(B) cyclobutane
(C) cyclopentane
(D) cyclohexane
4. Which of the following structures represents trans-1,3-dimethylcyclohexane?
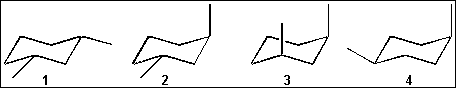
(A) 1
(B) 2
(C) 3
(D) 4
5. What is the IUPAC name for the following compound?
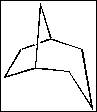
(A) bicyclo[5.4.3]octane
(B) bicyclo[3.2.1]octane
(C) bicyclo[3.2.1]hexane
(D) bicyclo[2.2.1]octane
6. Which one of the following structures represents a different compound from the other three?

(A) 1
(B) 2
(C) 3
(D) 4
7. Which of the following compounds can adopt a chair conformation in which there are no axial methyl groups?
(A) 1,1-dimethylcyclohexane
(B) cis-1,2-dimethylcyclohexane
(C) trans-1,2-dimethylcyclohexane
(D)
trans-1,3-dimethylcyclohexane
8. Which of the following statements is not true regarding the conformation of substituted cyclohexanes?
(A) ring inversion of cyclohexanes between two chair conformations takes place via a boat conformation
(B) substituted cyclohexanes are destabilized by 1,3-diaxial interactions
(C) the boat conformation of cyclohexane is more stable than the chair conformation
(D) the relative amount of two conformations of substituted cyclohexanes can be determined from the difference in strain energy
9. What is the approximate dihedral angle between the two chlorine atoms in a
chair conformation of
cis-1,2-dichlorocyclohexane?
(A) 0°
(B) 60°
(C) 120°
(D) 180°
10. Which of the following undergoes the most exothermic combustion?
(A) octane
(B) 2-methylheptane
(C) 2,2-dimethylhexane
(D) 2,2,3,3-tetramethylbutane
|