|
1. Which of the following statements is true?
(A) All mirror images are enantiomers
(B) All molecules that have stereocenter centers are chiral
(C) Isomers that are not superimposable on their mirror images are enantiomers
(D) Superimposable structural isomers are enantiomers
2. Which of the following is the definition of a meso compound? (A) A molecule with stereocenters which is chiral
(B) A molecule with stereocenters which is not chiral
(C) A diastereomer with no stereocenter centers
(D) A chiral compound with more than one stereocenter center
3. Which of the following structures is different from the other three? 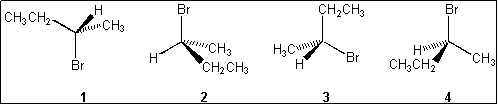
(A) 1
(B) 2
(C) 3
(D) 4
4. Which of the following compounds is/are chiral?
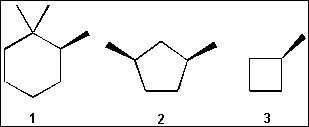
(A) only 1
(B) only 1 and 2
(C) only 2 and 3
(D) 1, 2 and 3
5. Which of the following Newman projections represents meso-2,3-dibromobutane?
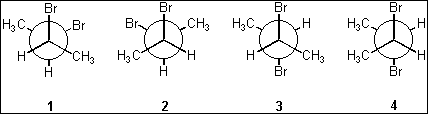
(A) 1
(B) 2
(C) 3
(D) 4
6. Which of the following structures represent the same stereoisomer?
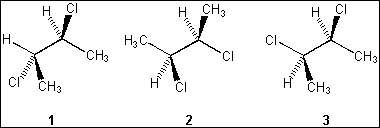 (A) only 1 and 2
(B) only 1 and 3
(C) only 2 and 3
(D) 1, 2 and 3
7. How many stereoisomers of 2,3-butanediol, CH3CH(OH)CH(OH)CH3, exist?
(A) 1
(B) 2
(C) 3
(D) 4
8. How many isomers (constitutional and stereoisomers) exist for dimethylcyclohexane?
(A) 3
(B) 5
(C) 6
(D) 9
9. A solution containing 0.2 g/mL of a pure R enantiomer in a 1 dm polarimeter rotates plane polarized light by +3°. What is the specific rotation of the R isomer?
(A) +0.06°
(B) +15°
(C) +67°
(D) +150°
10. A pure sample of the R-enantiomer of a compound has a specific rotation, [a], of -15°. A solution containing 0.6 g/mL of a mixture of enantiomers rotates plane polarized light by -3° in a 1 dm polarimeter. What is the enantiomeric excess
(%ee) of the mixture?
(A) 33% R
(B) 33% S
(C) 50% R
(D) 75% R
|