|
1. What is the value of the equilibrium constant, Keq, for the following
equilibrium?
[The pKa of HCl is –7. The concentration of water in a dilute aqueous
solution is 55 M]
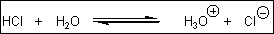
(A) 1.8
x 105 (B) 385
(C) 1.8
x 10-5
(D) -1.8
x 105
2.Which of the following is
the correct order of decreasing basicity (stronger base > weaker base)
(A) NH3 > MeNH2> H2O > HF
(B) MeNH2 > NH3 > MeOH > CH4
(C) NH3 > Me3N > H2O > MeOH
(D) CH3COONa > NaOH > NaOMe > NaNMe2
3. Which of the following compounds is the strongest acid?
(A) CH3COOH
(B) FCH2COOH
(C) ClCH2COOH
(D) BrCH2COOH
4. Which of the following compounds has the highest pKa?
(A) SiH4
(B) HCl
(C) H2S
(D) PH3
5. Which of the following terms describes the reactivity of boron tribromide, BBr3?
(A) Brønsted-Lowry acid and Lewis acid
(B) Brønsted-Lowry base and Lewis base
(C) Lewis acid and not a Brønsted-Lowry acid
(D) Lewis base and not a Brønsted-Lowry base
6. Which of the following terms describes the role of ethene in the acid-base reaction shown?
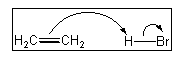 (A) Brønsted-Lowry acid
(B) Brønsted-Lowry base
(C)
Lewis acid
(D)
none of the above
7. Which of the following terms describes the role of ethanol in the acid-base reaction shown?
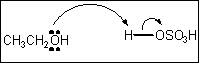 (A) Brønsted-Lowry
acid
(B) Brønsted-Lowry base
(C) Lewis acid
(D)
none of the above
8. Which species is the conjugate acid in the following acid-base reaction?
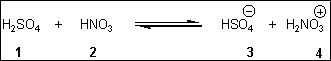 (A) 1
(B) 2
(C) 3
(D) 4
9. What is the value of the equilibrium constant, Keq, for the following reaction?
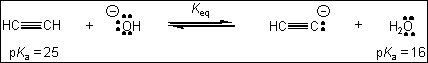 (A) 109
(B) 10-9
(C) 9
(D) 1/9
10. Which of the following is a feature of a Lewis acid?
(A) proton donor
(B) proton acceptor
(C) electron pair donor
(D) electron pair acceptor
|