|
1. The pKa of acetic acid, CH3COOH, is 4.76. What is the value of the
equilibrium constant Keq, for the following equilibrium?
[The concentration of water in a dilute aqueous solution is 55 M]?

(A)
3.2 x 107
(B)
3.15
(C)
3.2
x 10-7
(D)
--3.2
x 107
2. Which of the following compounds is the strongest acid?
(A) CH3COOH
(B) ClCH2COOH
(C) CH3CH2OH
(D) ClCH2CH2OH
3. Which of the following anions is the strongest base?
(A) CH3COO-
(B) HO-
(C) NH2-
(D) Cl-
4. Which of the
following compounds is the strongest acid? (A) CH3OCH3
(B) CH3CH2OH
(C) CH3CHO
(D) CH3CO2H
5. Which of the following is
present in the highest concentration upon dissolution of H2SO4
in water?
(A) H2SO4
(B) H+
(C) H3O+
(D) HO-
6. Which of the following
terms describes the role of ethanol in the acid-base reaction shown?
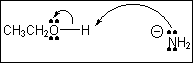
(A) Brønsted-Lowry acid
(B) Brønsted-Lowry base
(C)
Lewis base
(D)
none of the above
7. Which of the following terms describes the role of ethyne in the acid-base reaction shown?
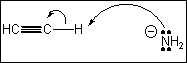
(A) Brønsted-Lowry acid
(B) Brønsted-Lowry base
(C)
Lewis base
(D)
none of the above
8. Which species is the conjugate acid in the following acid-base reaction?
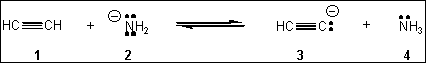
(A) 1
(B) 2
(C) 3
(D) 4
9. What is the value of the
equilibrium constant, Keq, for the following reaction?
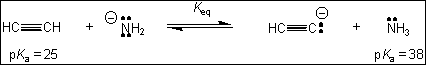
(A) 1013
(B) 10-13
(C) 13
(D) 1/13
10. Which of the following concepts can be used to rationalize
the observation that acetic acid is a stronger acid than methanol?
(A) electronegativity
(B) resonance
(C) valence shell electron pair repulsion theory
(D) Pauli exclusion principle
|