|
1. The reaction of
1-bromopropane with sodium iodide gives 1-iodopropane. What is the effect of
doubling the concentration of NaI on the rate of the reaction?
(A) the rate decreases by a factor of 2
(B) the rate decreases by a factor of 2
(C) the rate increases by a factor of 2
(D) the rate increases by a factor of 4
2. Which of the following
alkyl halides undergoes the fastest SN2 reaction with sodium cyanide,
NaCN?
(A) methyl iodide
(B) ethyl iodide
(C) 2-iodopropane
(D) tert-butyl iodide
3. Which of the following
alkyl halides undergoes the fastest solvolysis reaction with methanol, CH3OH?
(A) methyl chloride
(B) ethyl chloride
(C) 2-chloropropane
(D) tert-butyl chloride
4. What is the major organic
product obtained from the following reaction?
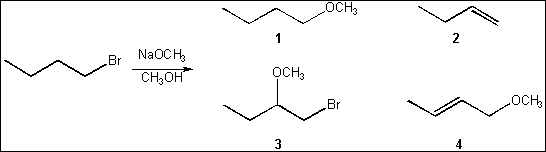
(A) 1
(B) 2
(C) 3
(D) 4
5. Which of the following
solvents is the best choice for the reaction of 1-chlorohexane with sodium
bromide?
(A) N,N-dimethylformamide
(B) water
(C) hexane
(D) toluene, PhCH3
6. Which of the following sets consists of only polar protic solvents?
(A) water, DMF, DMSO
(B) acetic acid, methanol, water
(C) DMSO, ethanol, acetonitrile
(D) DMF, acetonitrile, DMSO
7. Which of the following
energy diagrams represents the course of an exothermic SN2 reaction?
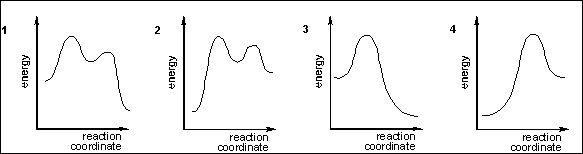 (A) 1
(B) 2
(C) 3
(D) 4
8. Which of the
following is not
a characteristic of SN2 reactions?
(A) the electrophilic carbon undergoes inversion of stereochemistry
(B) the rate is proportional to the concentration of substrate
(C) the rate is proportional to the concentration of nucleophile
(D) the rate is independent of the solvent
9. Which of the following
statements is true regarding the reaction of tert-butyl bromide with water?
(A) the rate is
proportional to the concentration of tert-butyl bromide
(B) the rate is
proportional to the concentration of water
(C) the rate is
independent of the identity of the solvent
(D) the rate of the
reaction is independent of the temperature
10. Which of the following compounds is the most nucleophilic in polar protic solvents?
(A) H2O
(B) NH3
(C) H2S
(D) PH3
|