|
1. Which of the following compounds gives an infrared spectrum with peaks at
3300 cm-1 (strong, broad peak) and 1640 cm-1
(sharp, weak peak)?
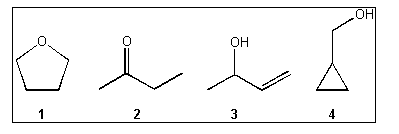
(A)
1
(B)
2
(C)
3
(D)
4
2. What is the multiplicity of the signal in the 1H NMR spectrum for the methyl protons of propane?
(A) singlet
(B) doublet
(C) triplet
(D) quartet
3. Which of the following
combinations of peaks appears in the 1H NMR spectrum of
1,2-dimethyoxyethane, CH3OCH2CH2OCH3?
(A) two singlets
(B) a singlet and a triplet
(C) a singlet and two triplets
(D) a doublet and a triplet
4. How many signals appear in the proton-decoupled 13C NMR spectrum of
bromobenzene?
(A) 3
(B) 4
(C) 5
(D) 7
5. Which of the protons in
the following molecule appear furthest downfield in the 1H NMR
spectrum?
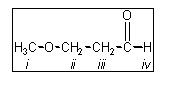
(A) i
(B) ii
(C) iii
(D) iv
6. The chemical shift of the
protons of acetone, CH3COCH3, is 2.1 ppm when the spectrum
is obtained at 250 MHz. What is the chemical shift of these protons when the
spectrum is acquired at 500 MHz?
(A) 1.05 ppm
(B) 2.1 ppm
(C) 4.2 ppm
(D) 8.4 ppm
7. What is the hydrogen
deficiency index for a compound with a molecular formula of C6H11N?
(A) 1
(B) 2
(C) 3
(D) 4
8. Which of the following
compounds gives a 1H NMR spectrum consisting of only two singlets?
(A) CH3OCH2CH2OCH2CH3
(B) CH3OCH2CH2CH2CH2OH
(C) CH3OC(CH3)2OCH3
(D) CH3OCH2CH(CH3)OCH3
9. Which of the following
compounds gives a 1H NMR spectrum consisting of only a singlet,
triplet and pentet?
(A) CH3OCH2CH2CH2CH2OH
(B) CH3OCH2CH2OCH2CH3
(C) CH3OCH2CH2CH2OCH3
(D) CH3OCH2CH(CH3)OCH
10. Which of the following compounds gives a peak in the IR spectrum at
approximately 1700 cm-1 and a singlet in the 1H NMR
spectrum at between 2 and 3 ppm? (A)
CH3CH2CH2CHO
(B)
CH3CH2COOCH3
(C)
CH3CH2COCH3
(D)
CH3CH2OCH2CH3
|