Step 1: Electrophilic Addition |
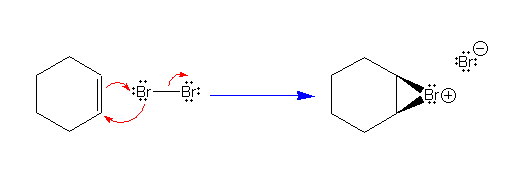 |
This flow of electrons forms the cyclic bromonium ion.
We have already seen this ion as the intermediate in the
addition of Br2 to alkenes to form a trans
1,2-dibromide. However, when used as a solvent, water will
act as a nucleophile. Click on an
electrophilic atom in the structure if the starting material
shown above. |
Step 1 - Step 2 - Step
3
|
|
|
|

