Step 2: Nucleophilic
Ring-opening |
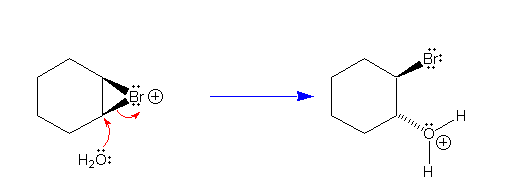 |
The ring-opening reaction is facilitated by relief of ring
strain in the 3-membered cyclic bromonium ion. A new
carbon-oxygen bond is formed by an SN2 reaction on the
electrophilic carbon atom in which the nucleophile (water)
approaches from the side opposite from the departure of a
leaving group (bromine). |
Step 1 - Step 2 - Step 3
|
|
|
|
Overall Reaction
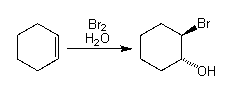
|
|
|
|

