Summary |
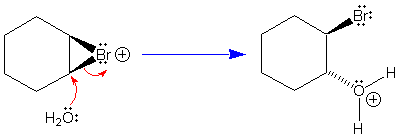
The key steps in the reaction of an alkene with Br2/H2O
are: (i) formation of the cyclic bromonium ion by syn addition of
bromine to the alkene, followed by (ii) nucleophilic ring opening of the
cyclic bromonium ion by an SN2 reaction in which water adds
to the back of the carbon-bromine bond to establish the
anti-stereochemistry of the addition (shown here).
Additional concepts:
Regiochemistry for formation of bromohydrins from unsymmetrically
substituted alkenes
Use of other nucleophilic solvents for brominations |
|
|
|
|
Key reaction: Nucleophilic attack of water
on cyclic bromonium ion
|
Overall Reaction
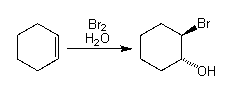
|
|

