|
RESEARCH
self-assembling polyphilic polymers |
|
| GIT > Chemistry and Biochemistry > Collard Faculty Page > Research | |
|
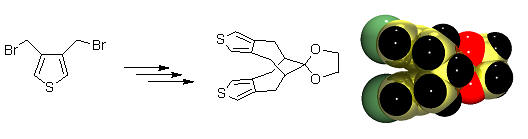
Pi-stacked Conjugated Oligomers Conjugated polymers display interesting conducting and semiconducting properties that make them interesting candidates for use in light emitting diodes (LEDs), field effect transistors (FETs) and sensors. The conductivity of doped conjugated organic polymers requires migration of delocalized charge carriers along conjugated chains and through pi-stacks. Oligomers with well-defined structures serve as useful models for conjugated polymers, which are often heterogeneous (polydisperse and semicrystalline). However, most physical-organic studies of the electronic structure of conjugated oligomers make use of linear polyenes as one-dimensional models, which can only be used to probe the delocalization and migration of carriers along individual non-interacting chains; thereby ignoring chain-chain interactions. We address this deficiency be preparation of covalently-stacked conjugated oligomers.
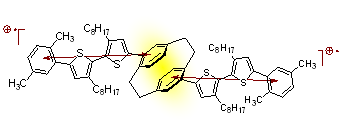
Example of an oligothiophene-substituted paracyclophane in which the terminal rings of tetrarene oligomers are held in a cofacial stack
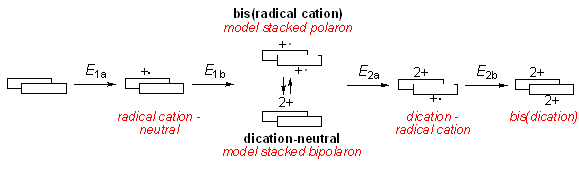
This approach makes use of multi-step organic synthesis in combination with electrochemical and spectroscopic characterization of the compounds. The stacked oligomers have a number of redox states relevant to conjugated polymers.
Representative publications
“Stacked Conjugated Oligomers as Molecular Models to Examine Interchain Interactions in Conjugated Materials”, K.M. Knoblock, C.J. Silvestri, D.M. Collard, Journal of the American Chemical Society, 2006, 128, 13680-13681.
"Pi-Stacked Conjugated Polymers: The Influence of Paracyclophane Pi-Stacks on the Redox and Optical Properties of a New Class of Broken Conjugated Polythiophenes", F. Salhi, D.M. Collard, Advanced Materials 2003, 15, 81.
"The Influence of Pi-Stacking on Redox Properties of Oligothiphenes: (α-Alkyloligothienyl)para[2.2]cyclophanes", F. Salhi, B. Lee, C. Metz, L.A. Bottomley, D.M. Collard, Organic Letters 2002, 4, 3195-3198.
|
|
|
Professor David Collard |
Office: 2100J Molecular Science and Engineering
|
|
|
CHEM home | GT disclaimer |